The role of EMG and kinematic measurements for objective symptom evaluation
Parkinson’s disease (PD) is a neurodegenerative disease without any curative treatment or neuroprotective or disease-halting drugs. It is characterized by motor symptoms that are still quantified mostly by subjective methods. Studies have shown that combining surface electromyography (EMG) and kinematic measurements can lead to objective quantification of a PD patient’s neuromuscular and motor function. Based on detailed symptom data, personalized treatment can be planned and optimized over the progression of the disease.
What is EMG and surface EMG?
The human motor system controls a person’s posture, force and movements. It consists of the central motor system and a large number of motor units (MUs). Each MU consists of a motor neuron in the spinal cord, the multiple branches of its axon and the muscle fibers it innervates. This creates a link between the central nervous system and the skeletal muscles, enabling human movements.
Electromyography (EMG) is a test used to measure and record the amount and timing of electrical activity produced by the skeletal muscles and the nerves that control them. An EMG detects issues with motor coordination, motor nerves, muscles or the communication between them.
The electrical potential caused by the muscle function is called the electromyogram, which is the graphical or visual representation of the electrical activity recorded during an EMG procedure. An EMG signal can be measured from muscles by using intramuscular needle electrodes or surface electrodes attached to the skin, which is known as surface EMG. [8, 9]
Figure 1: Motor system
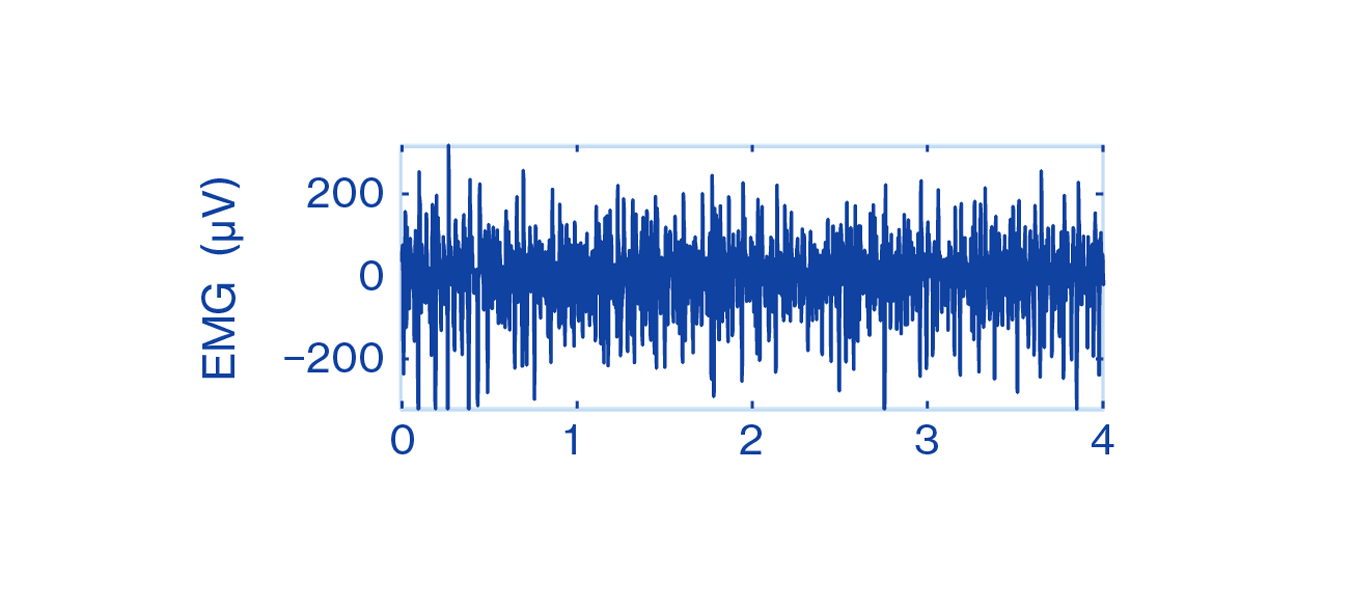
The EMG records the electrical muscle activity causing our movements. A muscle at rest has no electrical activity. Whenever a muscle contracts, a burst of electric activity is generated, which propagates through adjacent tissue and moves our bone. The EMG signals are impulse-like waveforms consisting of MU action potentials (MUAPs) in the vicinity of the measuring electrodes.
Common parameters analyzed from surface EMG signals are based on amplitude and spectral analysis. They quantify the level of muscle activation or fatigue.
EMG spike pattern holds essential information
EMG was first used to monitor the progress of a patient’s rehabilitation. Recently, it has also been widely used for the diagnosis of neurological and muscular pathology.
Still, EMG as a measurement method has had its own difficulties. MUAPs are impulse-like patterns or spikes. Therefore, the EMG signal appears as a messy, spikey-looking, impulse-like waveform that is complicated for untrained healthcare professionals to interpret. Collecting, processing and using recorded EMG signals for analysis has been challenging due to the vast amount of data that can be obtained with the measurement method.
Figure 2: EMG signal
In PD, the EMG spike pattern itself contains the essential information about the disease [8]. Therefore, conventional methods of EMG analysis that only look at amplitude and spectral parameters are not effective in analyzing EMG data measured from patients with PD.
Evaluating PD symptoms until now
There are four primary symptoms of PD: tremor, rigidity, bradykinesia and postural instability [5]. The diagnosis of PD is based on the presence of primary symptoms and the response to anti-parkinsonian medication [5]. Although there is no cure for PD, the symptoms can be relieved, for instance, with anti-parkinsonian medication or deep brain stimulation (DBS) [4].
Until now, motor impairment and treatment effects have been clinically evaluated subjectively using standardized rating scales, such as the Unified Parkinson’s Disease Rating Scale (UPDRS) [1, 3]. These provide the current golden standard in evaluating PD severity and tracking the disease’s progression [7]. This method is considered the first-generation approach.
Diagnostic accuracy by subjective clinical evaluation has been low. According to clinicopathological studies in the UK and Canada in 2006, accuracy was 75%, and at early stages of the disease, only 70% [11]. There has been, however, significant progress in the development of diagnostic biomarkers since.
Over the years, several more objective methods, considered the second-generation approach, have been proposed to improve the evaluation of symptoms in PD. This second-generation approach uses dedicated devices with motion sensors or accelerometer data from a smartwatch. These methods can capture movement but not the root cause of the movement originating from the brain through the motor nerves to our muscles and bones. Without knowing the root cause, it may be unclear if the movement is voluntary or involuntary and if it relates to a movement disorder or not.
Connecting EMG and kinematic measurements with PD
During the past two decades, there has been a growing interest in using the combination of EMG and kinematic measurements to understand better the control of human movements, movement disorders like PD and their treatment. A total of 249 articles have been published in scientific journals covering studies using EMG for PD in the past 10 years.
The combination of EMG and kinematic measurements, the third-generation approach, can revolutionize the accuracy of measured symptom data.
EMG has been used to objectively evaluate the different motor symptoms of PD and indicate how the disease modifies the muscle activity patterns during rest periods or during different types of movement. It provides valuable insight for making data-driven decisions about both muscle function and motor control that are affected by the disease. This helps to objectively evaluate the effects of different therapies or the variation in symptom severity at different points in time.
In 2006, a research study started at the University of Kuopio, today the University of Eastern Finland, in collaboration with clinical partners that used a novel mathematical method for combining EMG and kinematic data for the evaluation of PD symptoms. This combination of EMG and kinematic measurements enabled a breakthrough in discriminating between PD patients and healthy persons and the treatment effects at the neuromuscular level to be quantified. The research included nine different patient studies in Finland, the US, China and Russia. Over 200 patients and 100 healthy controls participated in the research.